Manipulating Large Molecule Files
Problem
You want to manipulate large molecule files.
Ingredients
|
Difficulty Level


Solution
This recipe discusses several examples that handle large molecule files:
All solutions presented here utilize the OEMolDatabase class that provides fast read-only random access to molecular file formats that are readable by OEChem TK.
Hint
The usage of OEMolDatabase is highly recommended when:
You want to manipulate molecule files without parsing the molecules.
You want to access molecules in a file randomly.
You want to handle a large set of molecules that can not be held in memory all at once.
Creating Molecule Database Index File
The first simple example shows how to create an index file by calling the OECreateMolDatabaseIdx function. The OEGetMolDatabaseIdxFileName function returns the name of the index file associated with the given molecule filename.
The index file of a molecule database stores file position offsets of the molecules in the file. Generating an index file can be expensive, but it can be created only once and then it can speed up the handling of large molecule files significantly.
1def CreateMolDatabaseIndexFile(ifname):
2 idxfname = oechem.OEGetMolDatabaseIdxFileName(ifname)
3
4 if os.path.exists(idxfname):
5 oechem.OEThrow.Warning("%s index file already exists" % idxfname)
6 elif not oechem. OECreateMolDatabaseIdx(ifname):
7 oechem.OEThrow.Warning("Unable to create %s molecule index file", idxfname)
Download code
moldb_create.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_create.py drugs.sdf
Running the above command will generate the drugs.sdf.idx molecule
database index file.
See also
Index files section in the OEChem TK manual
Counting Molecules
The first is a simple example that show how to count the number of molecules in a file. After initializing an OEMolDatabase object with an input stream, the OEMolDatabase.NumMols method returns the number of molecule records read from the file into the database.
1def MolCount(fname):
2
3 ifs = oechem.oemolistream()
4 if not ifs.open(fname):
5 oechem.OEThrow.Warning("Unable to open %s for reading" % fname)
6 return 0
7
8 moldb = oechem.OEMolDatabase(ifs)
9 nummols = moldb.NumMols()
10 print("%s contains %d molecule(s)." % (fname, nummols))
11 return nummols
Download code
moldb_molcount.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_molcount.py drugs.sdf
Running the above command will generate the following output:
drugs.sdf contains 6 molecule(s).
===========================================================
Total 6 molecules
When the drugs.sdf file is opened by the molecule database,
it automatically detects the presence of the corresponding index file drugs.sdf.idx.
In case when the index file exists, the number of file offsets stored in the index files
equal to the number of molecules stored in the corresponding drugs.sdf molecule file.
So returning the number of molecules stored in the drugs.sdf file does
not require to actually reading and parsing the molecules stored inside.
Output Molecule Titles
The next example outputs the title of the molecules stored in a file. After initializing an OEMolDatabase object, the molecules stored in the database can be accessed by their index. The title of all molecules can be retrieved by looping over the indices and calling the OEMolDatabase.GetTitle method that returns the title of molecule at the given index.
1def OutputMolTitles(ifs, ofs):
2 moldb = oechem.OEMolDatabase(ifs)
3
4 for idx in range(moldb.GetMaxMolIdx()):
5 title = moldb.GetTitle(idx)
6 if len(title) == 0:
7 title = "untitled"
8 ofs.write('%s\n' % title)
Download code
moldb_gettitles.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_gettitles.py drugs.sdf
Running the above command will generate the following output:
acetsali
acyclovi
alprenol
aminopy
atenolol
caffeine
Extracting Molecules by Title
The next example extracts compound(s) from a file based on a molecule title or a set of titles. This example uses the same OEMolDatabase.GetTitle method to access the title of the molecules. Molecules with matching title are then written directly to the output stream by using the OEMolDatabase.WriteMolecule method.
This example illustrates one of the main advantages of using molecule databases. Extracting molecules this way does not require actually parsing the molecules. The code below simply copies the bytes that encode the molecules from an input stream to an output stream without ever calling OEReadMolecule or OEWriteMolecule.
1def MolExtract(ifs, ofs, titleset):
2 moldb = oechem.OEMolDatabase(ifs)
3
4 for idx in range(moldb.GetMaxMolIdx()):
5 title = moldb.GetTitle(idx)
6 if title in titleset:
7 moldb.WriteMolecule(ofs, idx)
Download code
moldb_molextract.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_molextract.py -in drugs.sdf -title aminopy -out .sdf | python3 moldb_gettitles.py .sdf
Running the above command will generate the following output:
aminopy
Extracting Random Set of Molecules
It is a frequently occurring task to extract a random set of molecules from a large
molecule file. The code below first creates a random index set using the
random.sample() function.
The molecules corresponding to the indices are then copied over from the input
stream to the output stream.
The random access feature provided by the OEMolDatabase class makes this process very
fast.
Implementing the same process without OEMolDatabase class would either require
holding all molecules in memory at once or in the case of a very large molecule file
it would require reading the molecule file more than once.
1def RandomizePercent(ifs, ofs, percent, rand):
2 moldb = oechem.OEMolDatabase(ifs)
3
4 indices = range(moldb.GetMaxMolIdx())
5 size = max(1, int(percent * 0.01 * moldb.GetMaxMolIdx()))
6
7 mlist = rand.sample(indices, size)
8
9 WriteDatabase(moldb, ofs, mlist, size)
1def WriteDatabase(moldb, ofs, mlist, size):
2 for molidx in mlist[:size]:
3 moldb.WriteMolecule(ofs, molidx)
Download code
moldb_randomsample.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_randomsample.py -in drugs.sdf -out .sdf -p 50 -seed 1 | python3 moldb_gettitles.py .sdf
Running the above command will generate the following output:
aacetsali
atenolol
aminopy
prompt > python3 moldb_randomsample.py -in drugs.sdf -out .sdf -n 4 -seed 5 | python3 moldb_gettitles.py .sdf
Running the above command will generate the following output:
aminopy
caffeine
atenolol
alprenol
Splitting Molecule Database
The next example shows how to split a large molecule file into smaller pieces. There are two possible ways to do this.
The first function SplitChunk shows how to split a molecule into \(n\) sized chunks. Each output file is going to contain \(n\) number of molecules except the last one that will have the remainder.
1def SplitChunk(ifs, chunksize, outbase, ext):
2 moldb = oechem.OEMolDatabase(ifs)
3 chunk, count = 1, chunksize
4
5 for idx in range(moldb.GetMaxMolIdx()):
6 if count == chunksize:
7 ofs = NewOutputStream(outbase, ext, chunk)
8 chunk, count = chunk + 1, 0
9 count += 1
10 moldb.WriteMolecule(ofs, idx)
The second function SplitNParts shows how to spit a molecule file by determining the number of chunks rather than the size of the chunks.
1def SplitNParts(ifs, nparts, outbase, ext):
2 moldb = oechem.OEMolDatabase(ifs)
3 molcount = moldb.NumMols()
4
5 chunksize, lft = divmod(molcount, nparts)
6 if lft != 0:
7 chunksize += 1
8 chunk, count = 1, 0
9
10 ofs = NewOutputStream(outbase, ext, chunk)
11 for idx in range(moldb.GetMaxMolIdx()):
12 count += 1
13 if count > chunksize:
14 if chunk == lft:
15 chunksize -= 1
16
17 ofs.close()
18 chunk, count = chunk + 1, 1
19 ofs = NewOutputStream(outbase, ext, chunk)
20
21 moldb.WriteMolecule(ofs, idx)
Download code
moldb_molchunk.py and
drugs.sdf
supporting data file
Usage:
Splitting a molecule file into chunks of size \(n\)
prompt > python3 moldb_molchunk.py -in drugs.sdf -out chunk.sdf -size 4 | python3 moldb_molcount.py chunk*.sdf
Running the above command will generate the following output:
chunk_0000001.sdf contains 4 molecule(s).
chunk_0000002.sdf contains 2 molecule(s).
===========================================================
Total 6 molecules
Splitting a molecule file into \(n\) chunks:
prompt > python3 moldb_molchunk.py -in drugs.sdf -out chunk.sdf -num 4 | python3 moldb_molcount.py chunk*.sdf
Running the above command will generate the following output:
chunk_0000001.sdf contains 2 molecule(s).
chunk_0000002.sdf contains 2 molecule(s).
chunk_0000003.sdf contains 1 molecule(s).
chunk_0000004.sdf contains 1 molecule(s).
===========================================================
Total 6 molecules
Sorting Molecules
The last two examples show how to sort large molecule files. In the first example the sorting criterion is the molecule title of the input compounds. In the SortByTitle function, first a OEMolDatabase object is constructed with an input molecule stream. Then a list of (title - molecule index) tuples is constructed using the OEMolDatabase.GetTitles method that returns all the titles for the database. After sorting this list in alphabetical order, the indices are extracted and the molecules in the database are re-ordered by calling the OEMolDatabase.Order method. Subsequent calls to OEMolDatabase.Save method output the database in this new order.
1def SortByTitle(ifs, ofs):
2 moldb = oechem.OEMolDatabase(ifs)
3
4 titles = [(t, i) for i, t in enumerate(moldb.GetTitles())]
5 titles.sort()
6
7 indices = [i for t, i in titles]
8
9 moldb.Order(indices)
10 moldb.Save(ofs)
Download code
moldb_titlesort.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_titlesort.py drugs.sdf .sdf | python3 moldb_gettitles.py .sdf
Running the above command will generate the following output:
acetsali
acyclovi
alprenol
aminopy
atenolol
caffeine
The last example shows how to sort molecules by their molecular complexity. In the SortByComplexity function, first a OEMolDatabase object is constructed with an input molecule stream. Then by looping over all entries of the database, a list is generated that stores (complexity score - molecule index) tuples. After sorting this list (by the complexity scores), the indices are extracted and the molecules in the database are re-ordered by calling the OEMolDatabase.Order method. Subsequent calls to OEMolDatabase.Save method output the database in this new order.
1def SortByComplexity(ifs, ofs):
2
3 moldb = oechem.OEMolDatabase(ifs)
4
5 complexitylist = []
6
7 mol = oechem.OEGraphMol()
8 for idx in range(moldb.GetMaxMolIdx()):
9 complexscore = float("inf")
10 if moldb.GetMolecule(mol, idx):
11 oechem.OEPerceiveChiral(mol)
12 complexscore = oemedchem.OETotalMolecularComplexity(mol)
13
14 complexitylist.append((complexscore, idx))
15
16 complexitylist.sort()
17
18 indices = [idx for s, idx in complexitylist]
19
20 moldb.Order(indices)
21 moldb.Save(ofs)
Download code
moldb_complexitysort.py and
drugs.sdf
supporting data file
Usage:
prompt > python3 moldb_complexitysort.py drugs.sdf .sdf | python3 moldb_gettitles.py .sdf
Running the above command will generate the following output:
acetsali
caffeine
acyclovi
aminopy
alprenol
atenolol
Hint
The OEMolDatabase.Save method is optimized for the case whenever the output file format is the same as the input file format used to initialize the database. If the same file format is used, the molecule record’s bytes will be streamed directly, without an intermediate OEMolBase to do the conversion.
Discussion
The above examples perform various tasks of manipulating molecule files. Most of the scripts described here do no actually require fully parsing the molecules. The OEMolDatabase class was implemented for the purpose of performing these kind of operations very rapidly. Figure 1 illustrates the performance improvement that can be achieved when using molecule databases.
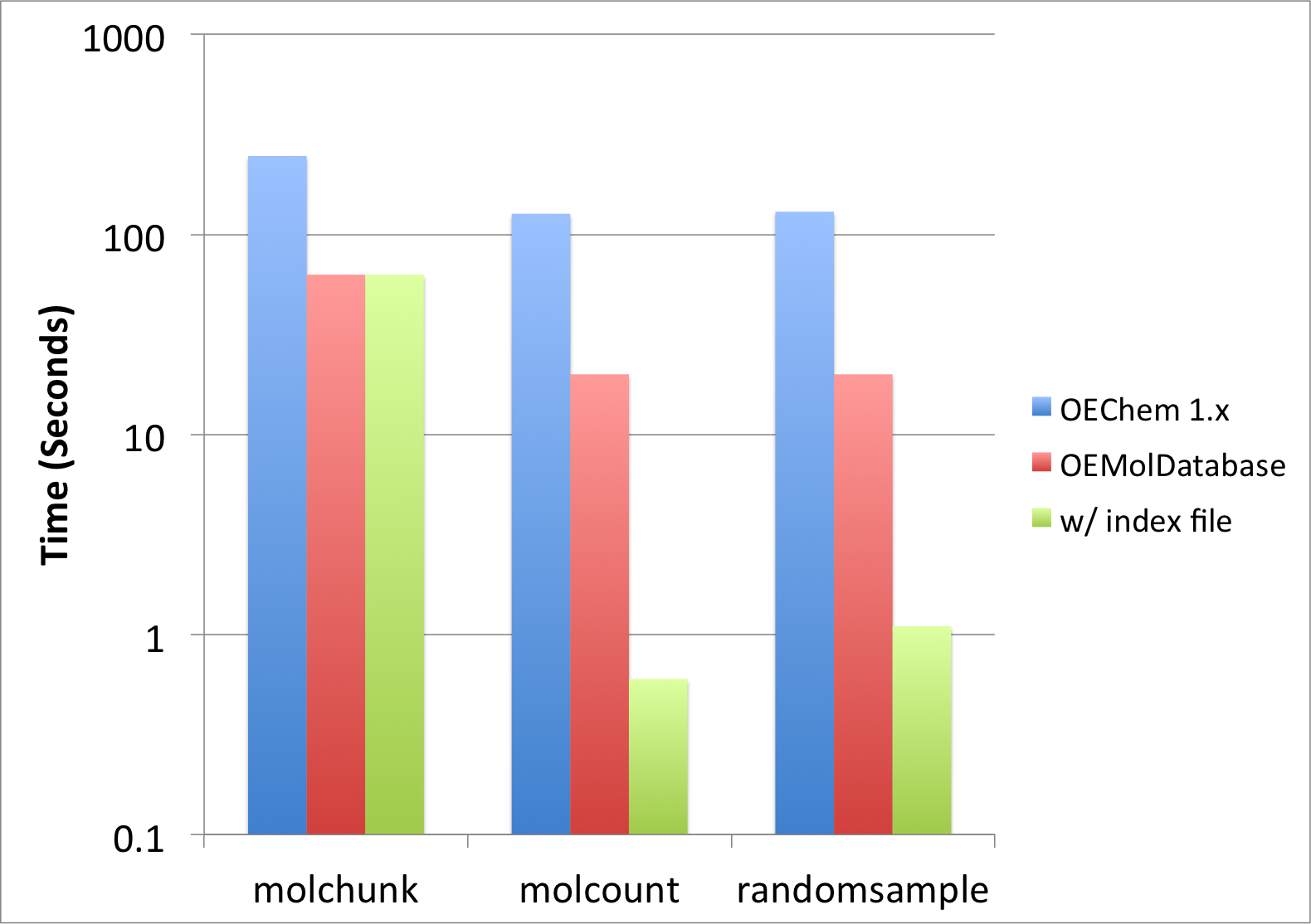
Figure 1: Performance improvement when using OEMolDatabase
The molecule file drugs.sdf (containing only 6 molecules) used in this recipe
to show the usage of the examples does not illustrate how awesome and
powerful molecule databases are.
See also
Rapid Similarity Searching of Large Molecule Files and Similarity Searching of Large Molecule Files recipes that show how to perform 2D similarity search on large molecule files.
See also in Python documentation
random.sample()
See also in OEChem TK manual
Theory
Molecular Database Handling chapter
API
OECreateMolDatabaseIdx function
OEGetMolDatabaseIdxFileName function
OEMolDatabase class
See also in OEMedChem TK manual
API
OETotalMolecularComplexity function