QUACPAC 1.7.0
Released October 2016
New features
-maxtime flag has been added to tautomer enumeration for setting time limits on searching. Its default value is 60 seconds.
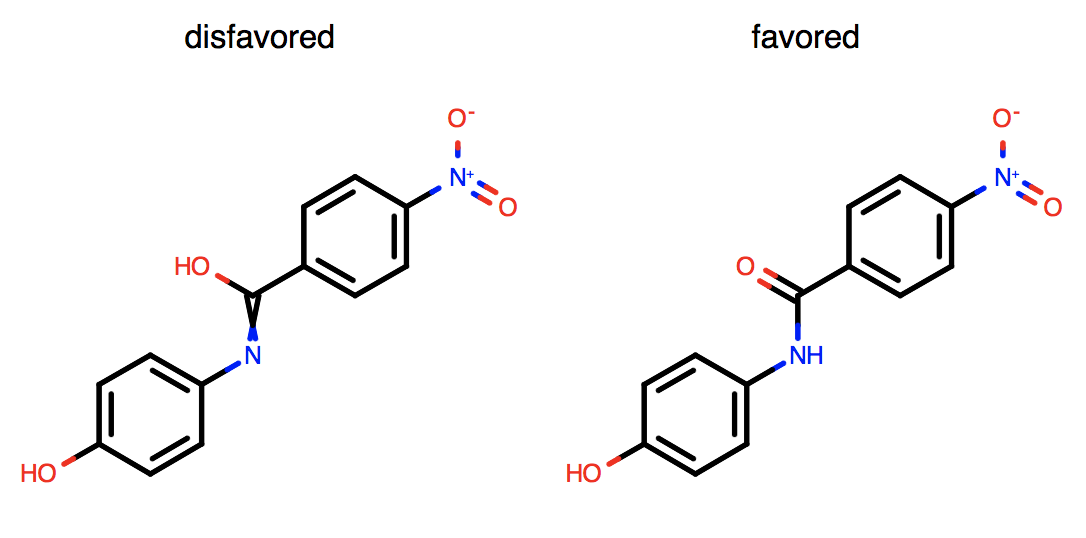
tautomers has been dramatically improved to provide a low-energy, medicinally relevant, “reasonable” tautomeric form that is suitable for depiction for chemists. Significant improvements have been made to the reasonable tautomer algorithm that affect its aliphatic and non-aromatic resonance portions. The depictions below are a useful guide for recognizing how tautomers are favored as reasonable:
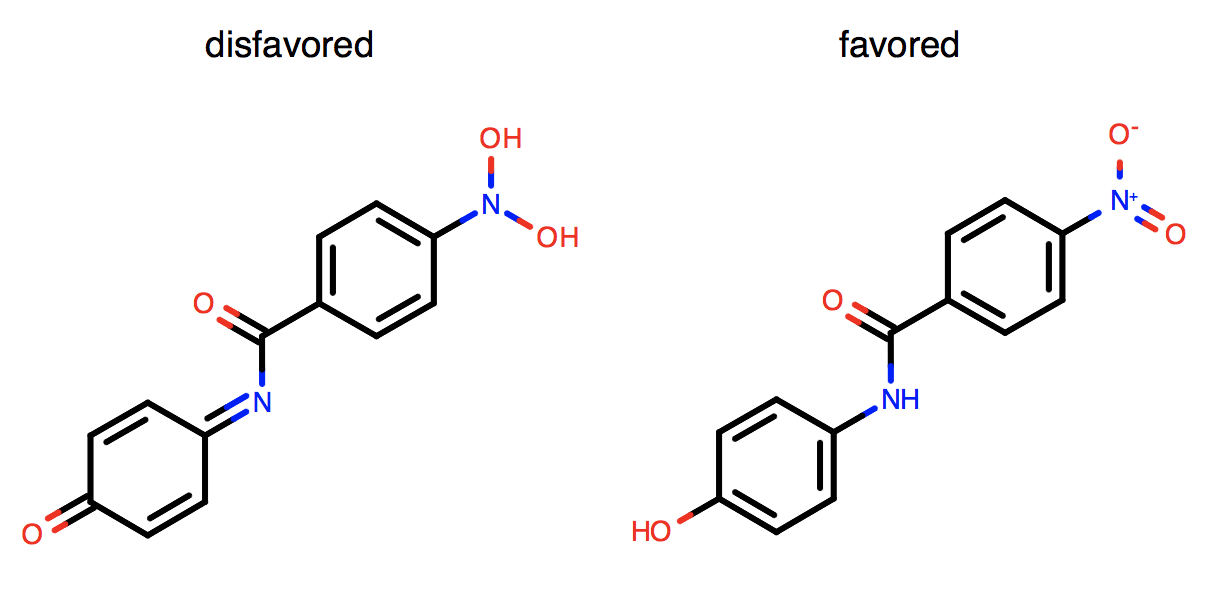
Conversion of carboxylates to diols and nitros to di-hydroxy amines is not favored.
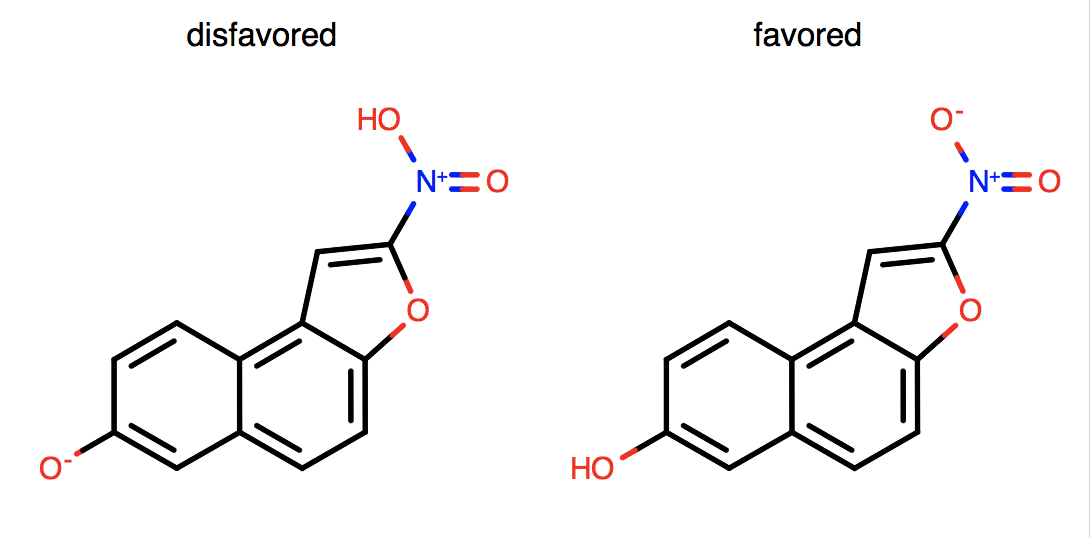
Generation of unnecessary, non-dative, formal charges is not favored.
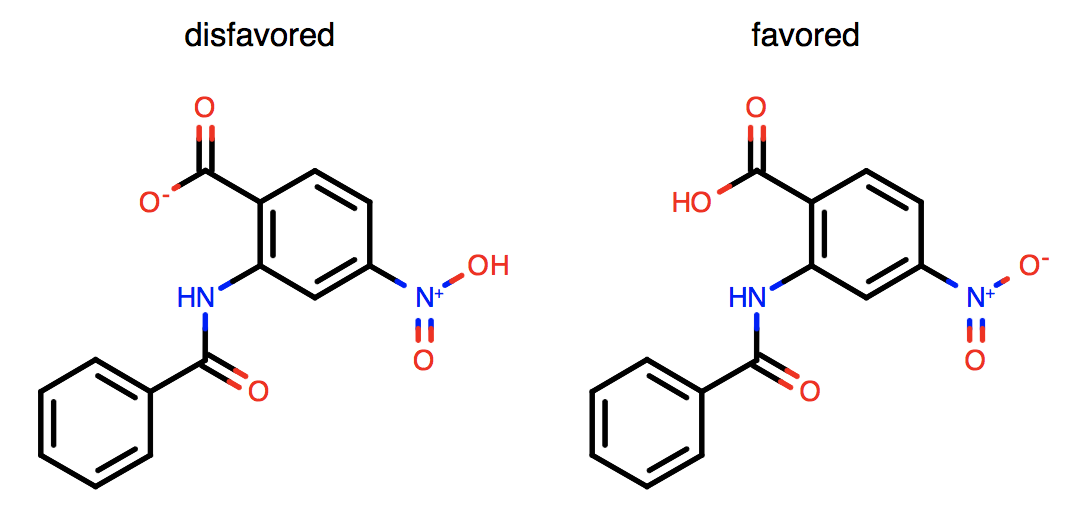
Exocyclic bonds adjacent to aromatic rings are accounted for.
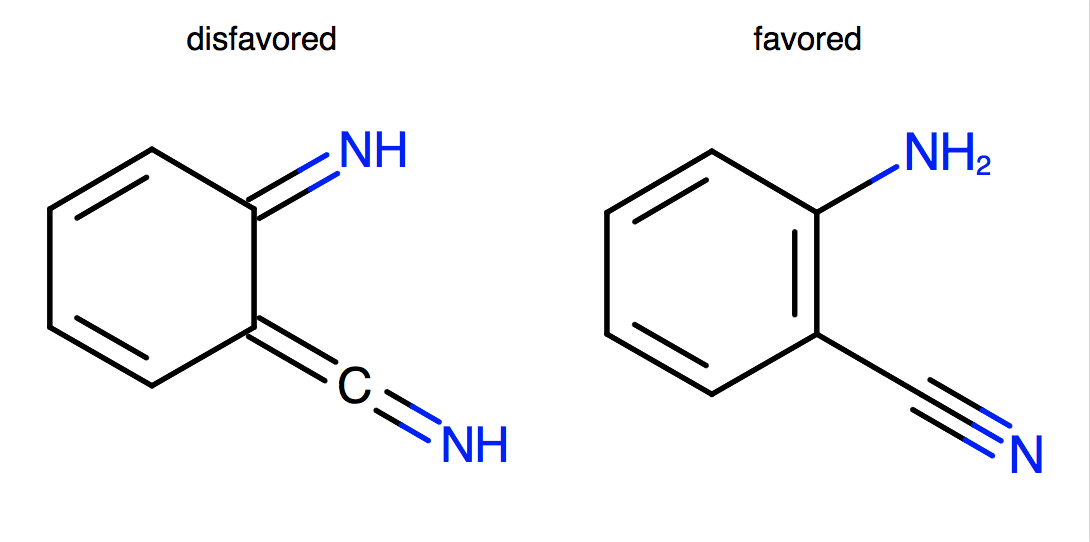
Priority is given to aliphatic double-bond positions.
AM1BCC ELF10, a new method for applying partial charges to a molecule, has been added to molcharge. AM1BCC ELF10 allows up to 10 diverse conformers to be selected from those having the Electrostatically Least-interacting Functional groups (ELF). These conformers are then charged with the AM1BCCSym method and the charge sets are averaged to come up with a single charge set that is applied to all conformers. This yields good quality charge sets even for charged molecules.
TIP3P water charges are now assigned when using Amber charge sets on molecules containing waters. The application of the other Amber charge sets to waters with explicit hydrogens will produce oxygen charges of -0.834 and hydrogen charges of +0.417.
The default value of -can flag for tautomers has been changed to true.
tautomers will output failed molecules to a fail file. The file will be named either tautomers.fail or, if the prefix is specified by -prefix flag, prefix.fail.
A log file has been added for molcharge, tautomers, and pkatyper.
tautomers, molcharge, pkatyper, and fixpka will now label unnamed input molecules as output_1, output_2, etc.
Bug fixes
molcharge now explicitly supports methods AM1BCCSym (a synonym for am1bcc) and AM1BCCSymspt (a synonym for am1bccspt).
Descriptions for molcharge methods have been updated in the usage documentation. In addition, a description for -formal has been added.
pkatyper no longer uses the deprecated OETyperMolFunction API.
tautomers no longer uses the deprecated OETautomerMolFunction API.
The wart symbol has been changed from “@” to “_” to help with parsing SMILES formatting.
Unbounded stack allocations have been removed from tautomers.
amberff94 will no longer identify a CYS residue as anionic when it is bonded by a sulfur to something other than another CYS residue.
pkatyper no longer outputs an empty file if the output format is not supported.
Other changes
opls method has been removed from molcharge.